Hydrated magnesium sulphate mgso 4 7h 2 o better known as epsom salt was discovered in 1618 by a farmer in epsom england when his cows refused to drink the water from.
Magnesium hydroxide state at room temperature.
It occurs in nature as the mineral brucite.
When submerged in water hydrogen bubbles form slowly on the surface of the metal though if powdered it reacts much more rapidly.
When combined with water h 2 o magnesia forms magnesium hydroxide mg oh 2 better known as milk of magnesia which is commonly used as an antacid and as a laxative.
It is a white solid with low solubility in water k sp 5 61 10 12.
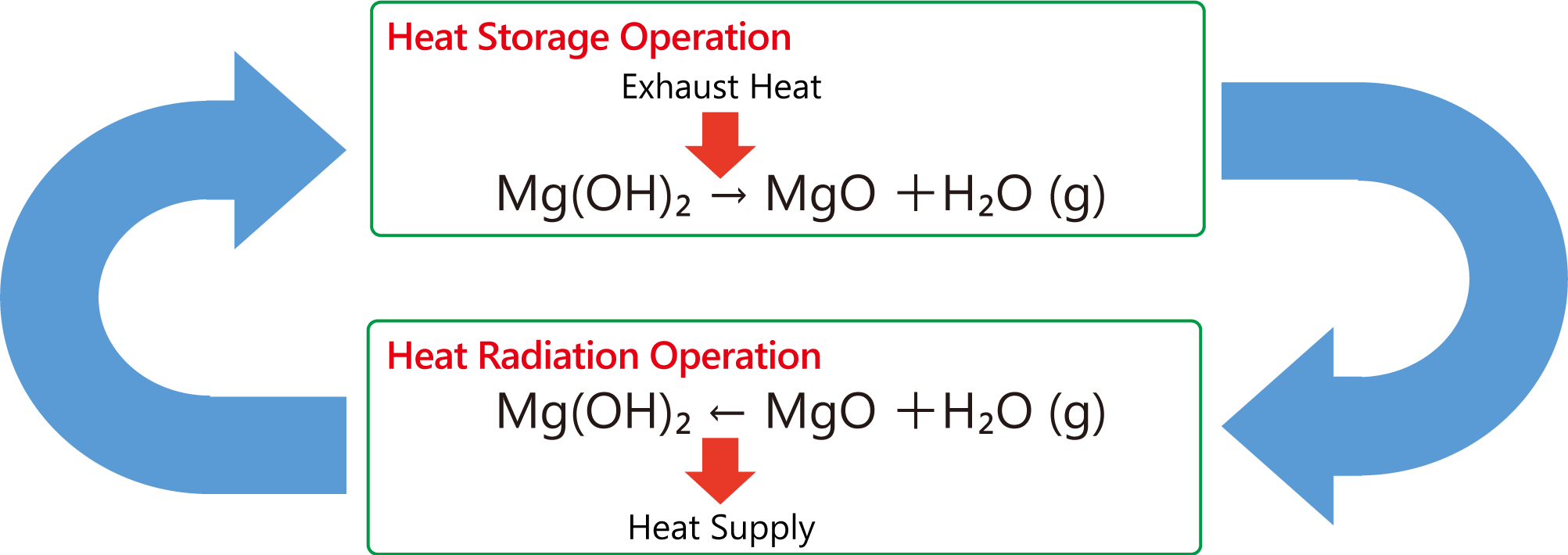
The principal phases of magnesium in seawater are chlorides and sulphates and the production process is initiated by the addition of a strong base to facilitate the precipitation of magnesium hydroxide mg oh 2 followed by thermal decomposition e g.
Magnesium hydroxide is added to plastics to make them fire retardant.
Hygroscopic substances absorb water molecules spontaneously when exposed to air.
Carbon dioxide is a gas at room temperature but a solid at low temperatures.
Carbon dioxide is a gas at room temperature but a solid at low temperatures.
Magnesium hydroxide is a common component of antacids such as milk of magnesia as well as laxatives.
Magnesium reacts with water at room temperature though it reacts much more slowly than calcium a similar group 2 metal.
Magnesium oxide is used in some antacids in making crucibles and insulating materials in refining some metals from their ores and in some types of cements.
Magnesium hydroxide is a solid at room temperature.
The reaction occurs faster with higher temperatures see safety precautions.
Magnesium oxide is used to make heat resistant bricks for fireplaces and furnaces.
Magnesium oxide is hygroscopic 1 2.
A familiar compound that exhibits this property is sugar.
Relative atomic mass.
Do not use magnesium hydroxide for longer than 7 days without medical advice.
Magnesium hydroxide is a solid at room temperature.
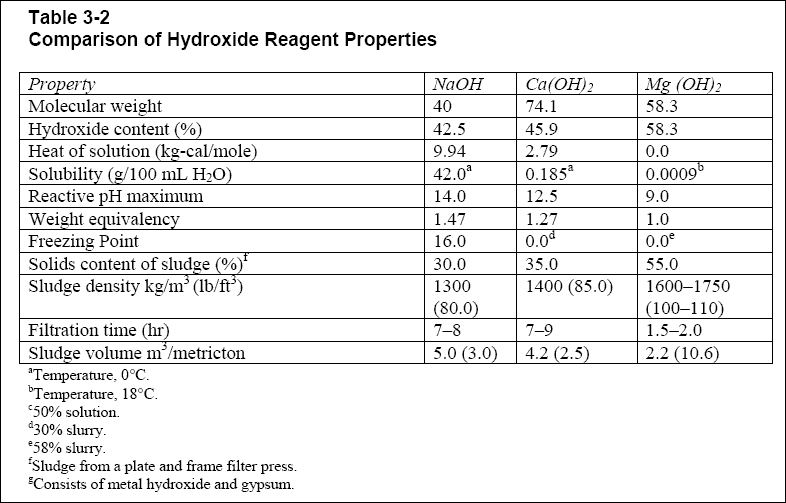
Magnesium hydroxide is the inorganic compound with the chemical formula mg oh 2.
When combined with water h 2 o magnesia forms magnesium hydroxide mg oh 2 better known as milk of magnesia which is commonly used as an antacid and as a laxative.
What is the physical state of copper hydroxide at room temp.
The oxidation state of an atom is a measure of the degree of oxidation of an atom.
Magnesium oxide s physical state at room temperature is a white odorless powder 2.